Abstract
Introduction
Hemophilia A is a rare bleeding disorder characterized by coagulation Factor VIII (FVIII) deficiency. Symptoms are primarily bleeding episodes that occur spontaneously or following injury, trauma, or surgical procedure. The treatment for hemophilia A involves replacement of FVIII for on-demand or prophylactic care. The aim of this analysis was to describe treatment outcomes and patterns between patients with hemophilia A who switch to emicizumab (Hemlibra®), Fc fusion protein (rFVIIIFc; Eloctate®), or other factor therapy using specialty pharmacy data from the US.
Methods
This was a retrospective observational descriptive study conducted between November 2014 and January 2021 using specialty pharmacy data including over 5,000 total patients with hemophilia A in the US. Patients included in the analysis were male, had a diagnosis of hemophilia A without a history of inhibitors, and had a minimum of 6 months baseline pre-index data and 6 months follow-up data post-index. Index date was defined as the first dispense of emicizumab after October 2018 or rFVIIIFc after May 2015. Patient reported and claims-based treatment outcomes assessed pre- and post-treatment included: annualized bleeding rate (ABR, calculated from patient bleed logs), any factor usage including on-demand FVIII use, prescribed and dispensed dosage of treatment, and frequency. Wastage as a percentage was a calculated as: (prophylaxis logged - prophylaxis prescribed)/prophylaxis dispensed.
Results
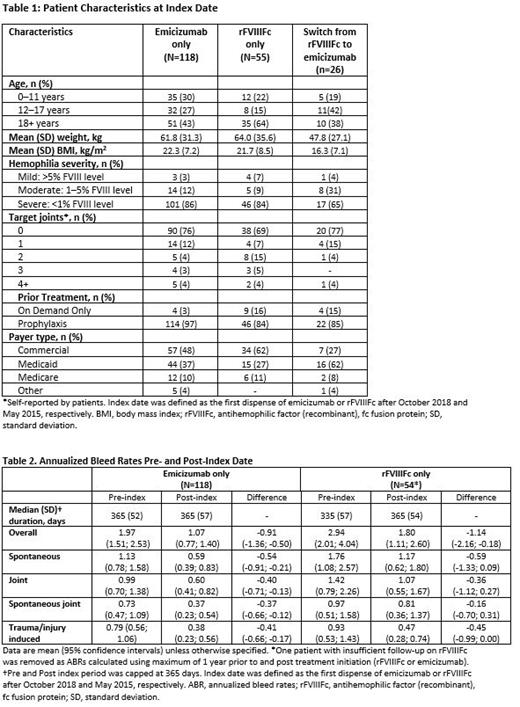
118 patients treated with emicizumab and 55 patients treated with rFVIIIFc were included in the analysis. Additionally, 26 patients switched from rFVIIIFc to emicizumab, and therefore met inclusion criteria for both treatment groups (these patients are described separately). Baseline characteristics are reported in Table 1; the majority of patients in all cohorts had severe hemophilia and prior prophylaxis therapy.
The most frequently prescribed dosing patterns for patients in the emicizumab cohort was once every 2 weeks (Q2W) for 56 patients (47.5%) and once weekly (Q1W) for 51 patients (43.2%). Whereas the most frequently prescribed dosing patterns for the rFVIIIFc only cohort was twice per week (BIW) in 27 patients (49.1%), every 4 days (Q4D) in 15 patients (27.3%), and Q1W in 2 patients (3.6%).
Total mean (95% confidence intervals [CI]) weekly prophylaxis consumption was 1.68 (1.65; 1.72) mg/kg in the emicizumab cohort and 83.17 (72.06; 94.29) IU/kg in the rFVIIIFc cohort post index. Mean (95% CI) weekly dispensed dosage for prophylaxis was 1.81 (1.76; 1.86) mg/kg emicizumab and 83.83 (72.55; 95.11) IU/kg rFVIIIFc post index. Mean (95% CI) total supplied FVIII for on-demand factor on hand was 5.14 (2.92; 7.35) IU/kg in the emicizumab cohort and 11.76 (9.06; 14.46) IU/kg in the rFVIIIFc cohort post index. Weekly percentage of supplied dosage wasted was 6.03% (4.50; 7.57; p<0.01) emicizumab for the overall cohort, with a mean (standard deviation [SD]) of 10.46% (12.21) wasted per patient within the emicizumab age <12 years cohort. Weekly percentage of supplied dosage wasted was 0.70 (-0.10; 1.50; p=0.09) rFVIIIFc for the overall cohort, with a mean (SD) of 2.63% (3.53) wasted per patient within the rFVIIIFc age <12 years cohort.
Mean (95% CI) change in overall ABR pre- and post-treatment was -1.14 (-2.16; -0.18) in the rFVIIIFc cohort and -0.91 (-1.36; -0.50) in the emicizumab cohort (Table 2). Mean (95% CI) changes in spontaneous ABR and spontaneous joint ABR pre- and post-treatment were -0.36 (-1.12; 0.27) and -0.16 (-0.70; 0.31), respectively, in patients treated with rFVIIIFc, whereas within the emicizumab group they were -0.40 (-0.71; -0.13) and -0.37 (-0.66; -0.12).
Conclusion
Patients were prescribed emicizumab labeled dosing primarily on a Q1W or Q2W pattern, whereas patients prescribed rFVIIIFc had more individualized dosing primarily being prescribed Q4D or BIW. FVIII for on-demand treatment use was found post index in both the factor and non-factor treated cohorts. Overall product wastage with emicizumab is higher than previously reported. Product wastage was highest among emicizumab patients under 12 years, but wastage was still considerable in patients 12 or older. This descriptive analysis indicated that both treatments remained effective with lower bleed rates than prior FVIII therapies.
Cockerham: Sanofi: Current Employment, Current equity holder in publicly-traded company. Wilson: Sanofi: Current Employment, Current equity holder in publicly-traded company; Alexion: Current equity holder in publicly-traded company. Frick: Trio Health, Inc.: Current Employment; Sanofi S.A.: Research Funding. Dasmahapatra: Sanofi: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal